Innovative. Intuitive. Integrated.
Collect data directly from your participants at any time and from any location, using any device.
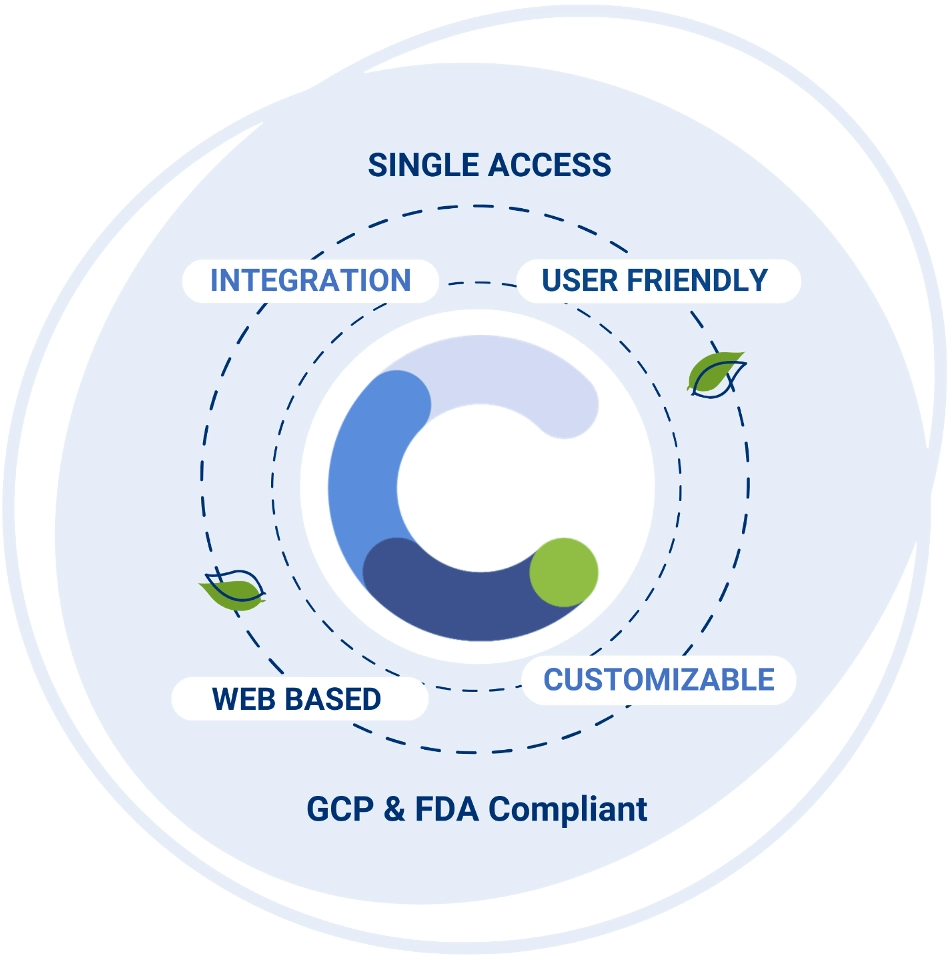
Meet Clinical.net
Transform your clinical trial journey with a customized approach! Use specific tools to speed up getting your treatment to market!
Transforming Clinical Trials with Seamless Data Sharing
Clinical.net stands out for our seamless approach to navigating the complexities of clinical trials. By facilitating effortless sharing of trial data at every stage, we not only accelerate clinical development but also enhance compliance, leading to a swifter path to market success. Our commitment to innovation and efficiency sets us apart in the industry, empowering our clients to achieve their goals with ease and confidence.
Embark on Your Path to Successful Clinical Trial Management
From project design to database lock, the proprietary software enables proactive study start-up management, direct electronic entry, information retrieval, and data analysis, thus ensuring complete transparency across a project’s lifecycle. Our expert team will guide you through every step of the process, ensuring seamless execution.
Discover the power of Clinical.net EDC platform!
Learn more about our Modules!
Consistent Data. Reliable Results.
Years of Expertise
%
Customer Satisfaction
Studies
Countries
Clinical.net – the truly global solution!
Reliable Results, Every Time.
Explore more about our expertise and capabilities.


