Cell & Gene Therapy Modules
Cell and gene therapy (CGT) is an innovative approach to treating various diseases by harnessing the power of cells and genes to address underlying genetic or cellular defects.
This form of therapy relies on the use of cells or genes to treat or prevent diseases, often providing personalized treatments that target directly the underlying cause of the syndrome. It involves modifying or introducing genetic material into a patient’s cells to correct or replace defective genes, enhance immune responses, or restore normal cellular functions. This cutting-edge therapy holds great promise for treating a wide range of diseases, including genetic disorders, cancers, and degenerative diseases.
The Cell and Gene Therapy Module (CGT) is a comprehensive solution within our clinical research platform Clinical.net, designed to support the development of clinical trials and to address the unique complexities and demands of cell and gene therapy trials. This module offers tailored solutions to support the entire lifecycle of CGT studies, from initial planning and protocol design to execution, monitoring, and data management, to meet the unique challenges and requirements of cell and gene therapy studies.
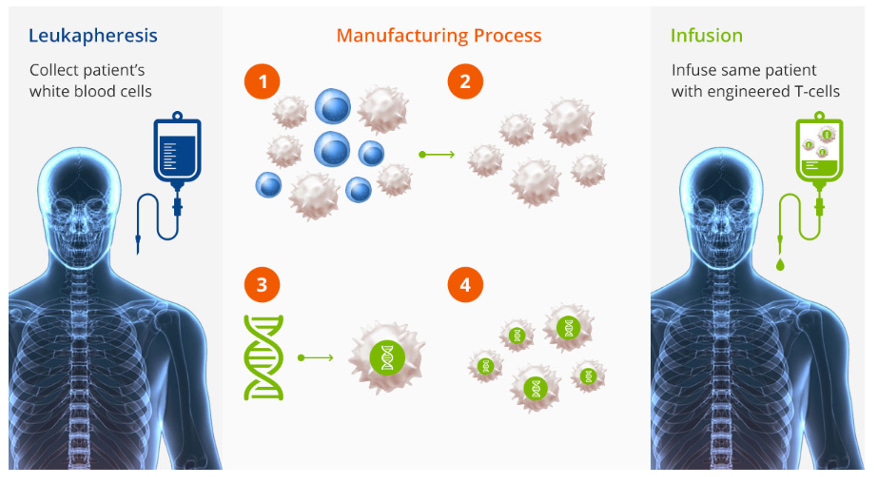
Cell Therapy Example: CAR-T CELL Process
Our Technology
Our advanced technology within Clinical.net serves as a fundamental ally for conducting successful CGT studies, offering an intuitive platform that supports the unique needs of clinical trials studies. Through our innovative technology, we aim to optimize every phase of study development, ensuring a seamless and efficient experience for investigators, sponsors, and patients involved. With a focus on customization and flexibility, we are committed to providing tailored solutions that allow sponsors to maximize the therapeutic potential of cell and gene therapies while enhancing the overall experience of investigators and patients involved.
Our Cell and Gene Therapy module within Clinical.net is enriched by two powerful tools:
- the Patient Allocation Tool
- the Dosage Calculation Tool
Which play a crucial role in optimizing and managing CGT studies.
Patient Allocation Tool
It allows for optimal patient distribution across different phases of clinical trials, ensuring strategic placement based on clinical appropriateness and economic convenience.
Integrated into our advanced technology, this tool provides an intuitive solution for facilitating patient allocation, contributing to optimizing study outcomes and maximizing overall research efficiency.
Additionally, this tool is particularly effective in studies with Umbrella trials, which allow simultaneous testing of multiple variants of a given product in a single patient population, enabling a comprehensive evaluation of CGT therapies in a broader clinical context and facilitating better understanding of outcomes.
Dosage Calculation Tool
Performs a crucial role in clinical decision-making by enabling dynamic adjustment of dosing assignments in real-time.
Integrated into our platform, it provides study teams and sponsors with immediate visibility into assigned dosages, ensuring timely communication and precise dose management for every patient involved in CGT studies.
This tool offers a reliable solution for optimizing therapeutic regimens and ensuring patient safety during treatment.
Key features of the Cell and Gene Therapy Module include:
Streamlined Sponsor-to-Site Interaction
Enhances communication and collaboration between sponsors and clinical trial sites, particularly for complex ATMP studies.
Adherence to Study Requirements
Ensures strict adherence to study protocols and requirements, minimizing deviations and safeguarding trial integrity.
Customization and Flexibility
Offers unparalleled flexibility, allowing customization of trial parameters to suit specific study needs and objectives.
Regulatory Compliance
We prioritize regulatory compliance by ensuring adherence to global standards and guidelines set forth by regulatory bodies such as the Food and Drug Administration (FDA) and the Center for Biologics Evaluation and Research (CBER). This ensures that all trial activities are conducted in accordance with regulatory standards, which is essential for sponsors conducting CGT trials to ensure their products meet rigorous safety, efficacy, and quality standards.
Real-Time Monitoring and Safety Reporting
Our module provides real-time monitoring capabilities, allowing investigators and sponsors to track study progress, patient safety, and protocol deviations promptly. It includes built-in safety reporting features to facilitate the timely reporting of adverse events and ensure regulatory compliance.
Data Capture and Management
With user-friendly interfaces and advanced data capture tools, our module simplifies data collection and management processes. It supports electronic data capture (EDC), enabling seamless integration with other data sources and systems for comprehensive data management.
Collaboration and Communication
Our module fosters collaboration and communication among study stakeholders, including investigators, sponsors, and regulatory authorities. It includes built-in communication channels and document sharing capabilities to facilitate efficient collaboration and decision-making.
Overall, our Cell and Gene Therapy Module is designed to streamline and optimize the conduct of CGT trials, enabling sponsors to accelerate the development and commercialization of innovative cell and gene therapies for patients in need, while ensuring patient safety and data integrity.

